By. Brian Acheson, Chelmsford High School
How can students learn about engineering while engaging in a discussion about their impact on the world? Introducing empathy into the design of a simple water filter can get teenagers to think not only about themselves but about someone on the other side of the world.
In 2009, Chelmsford High School (Chelmsford, Massachusetts) launched an Environmental Science course to provide an opportunity for students who were looking for an elective fourth year science course. As a school, Chelmsford emphasizes teaching students 21st century learning skills, with goals including sustainability. The Environmental Science course brings the concepts of sustainability to life for students at Chelmsford.
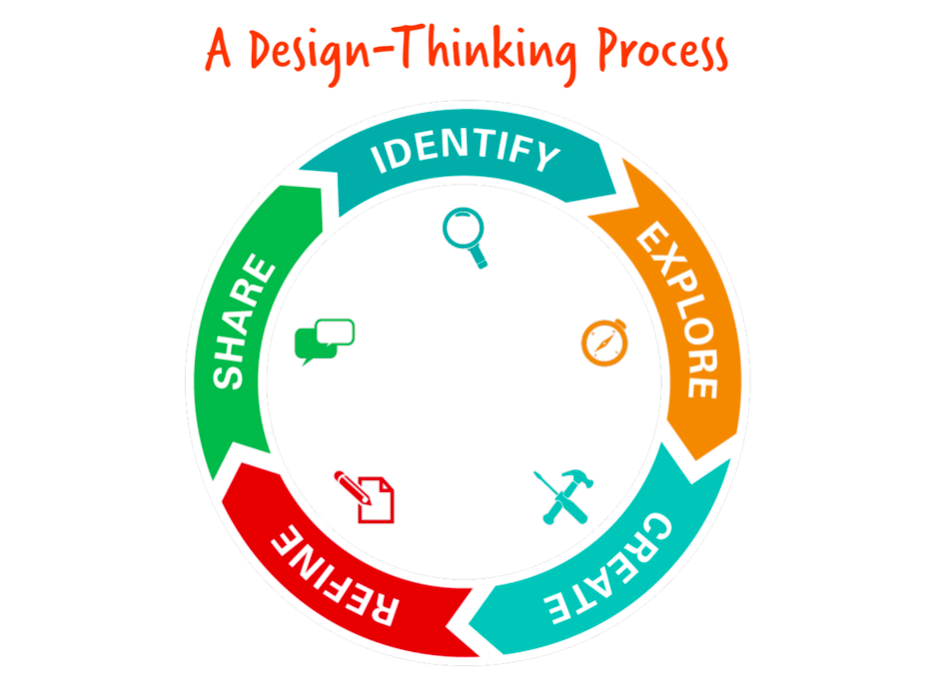
Over the last four years, Chelmsford’s Environmental Science course has blossomed through the use of resources provided by EcoRise. EcoRise’s K-12 program includes a robust curriculum that teaches sustainability, design innovation, and social entrepreneurship. The EcoRise curriculum challenges students to think about how their everyday lives affect the environment and how they can make changes, both big and small, to decrease their impact on the Earth for a better future. Through EcoRise’s Design Studio, students use a step-by-step process to solve problems through identifying, exploring, creating, refining, and sharing ideas. This comes in handy when trying to engineer solutions to sustainability problems.
One of the ways in which engineering and sustainability are brought into the classroom is through a water quality unit. The unit starts with students testing local water sources and supply methods. As the unit progresses, the view becomes more global. Students learn about the supply and demand of clean water. The final lesson allows students to take what they have learned about clean water and see how they might make a global impact. Students learn about watersheds from around the world, then use that information to create solutions to the problems the watersheds’ inhabitants face. The design method plays a central role throughout this lesson and the main hook that keeps students engaged is the empathy they develop for people they have never met, yet who are tied to each step of the process.
Each student is asked to identify a public water source from outside North America. Rivers are the most popular choice as they serve many functions, from acting as a local source for drinking water to playing an important role in commerce through transportation. Each student learns about all the uses of this water source:
- What do the locals use it for?
- How many people rely on this water source?
- What does industry use it for?
- How is it regulated?
- What are some of the known contaminants?
- What effect do these contaminants have on indigenous species?
- What are the origins of these contaminants?
- Are there any current measures being taken to resolve these contaminants? Is so, what?
Next, students are split into heterogeneous groups of three. The students are assigned in a way that gives each group a good mix of water sources from different continents as well as students of varying abilities. Together, group members explore the challenges that people face with each of the water sources. The groups begin to surmise that many of the problems are the same as those faced here in their hometown. Pollution through agriculture, industry, and local neglect drive up contaminants in the water. Many of the local contaminants were identified through testing in a water quality audit.
Armed with the knowledge of what contaminants are present in these water sources, the groups are presented with an engineering problem: How to clean up and remove the contaminants? To solve this problem, the groups are challenged to design, create, and test a filtration device that could be used by an individual living near one of the water sources identified in their research. The project goal is for each group to design a filter that can provide enough water to meet the needs of an individual for one day.
Before groups get to work building their filters, students are given the choice to assign roles to keep the project on task and focused. Suggestions are provided to the students (as recommended by EcoRise) for specific positions for each group member. For example, one member might focus on making sure the plans for constructing the filter meet the requirements, while another student might be responsible for keeping records for the group. A third person might be more inclined to be a team leader. Each group figures out, rather quickly, how best to work together to create a filter that meets all the specifications to produce pure water.
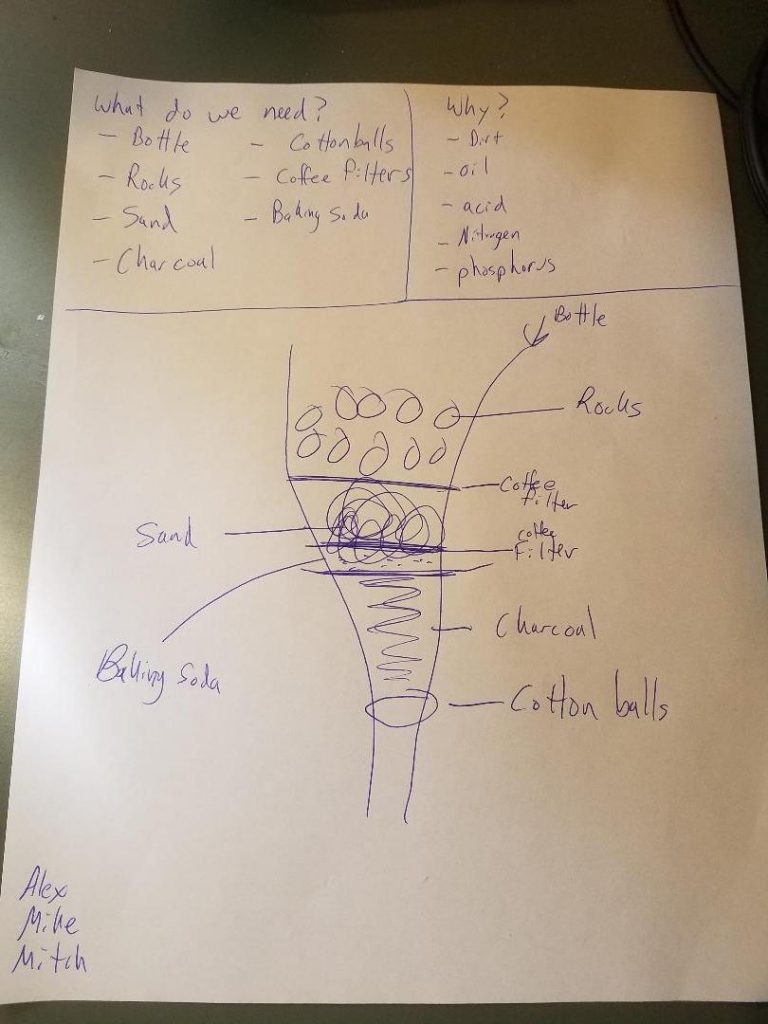
Research begins with groups figuring out what is needed to remove things like solid particulates, bacteria, excess nitrogen, iron, or even benzene from public water sources. Internet research is the biggest source of information for most of the groups. Many even find procedures for constructing filters. Some groups jump on to the first design they see without first determining if it is the right solution to purify all the possible contaminants.
Because the challenge is to construct a water filter that would sustain a human being for one day’s worth of water, the groups have to identify the amount of water needed for one person to live. Students normally turn the question inward and do a self-assessment. “What do we use water for each day?” The volume of water used is normally pretty high. When redirected to focus on what volume of water is needed to live, the students’ eyes open wide.
Once the filter design and components are identified, the groups set to work constructing their water filters. Most filter designs found online start with a layer of gravel, followed by a layer of sand, and end with a layer of activated charcoal. Students seemed to be surprised to find how basic components found in our soil are best suited for designing a filtration system. Side conversations typically occur within each group about how the earth we walk on is such a valuable resource, and not just for growing plants.
Charcoal, sand, and gravel are not the only things the groups will try to use to purify water. Many designs go too far, almost to the point of distillation. Occasionally, the groups need to be wrangled into a more realistic view of what makes good, usable water. A review of our water quality index work from earlier in the unit helps refocus efforts.
Some of the more interesting ideas that groups are allowed to run with include adding chemicals such as sodium bicarbonate. This leads to a great discussion on chemistry. Students will bring up the point that you are adding something else to the water, not really removing the contaminants. Also, once the initial water volume flows through the chemical, what happens to the sample? The concentration effect of the chemical on the initial flow is going to be greater than the effect on the later flow of water. Design-wise, do you always have to use the same amount of water in one of these filters to ensure that the chemical is really working appropriately? Do you have to collect all the processed sample in one large container? Can you reuse this filter, or do you have to restock it with the chemical?
Another idea many of the groups raise to improve water quality is to boil the water to kill unwanted bacteria. These groups are then challenged to research and propose a way to perform this task based on the existing resources available to inhabitants of the watersheds in their original water sources research. Many plans involve the simple use of fire, but other groups come up with alternative ways to heat the water. Solar radiation is a frequently chosen method.
After designing their filter, each group is then required to build a working model of their filter. Here is where groups will experience a lot of trial and error in their designs. Not only are the groups challenged to make filters for maximum quality, but cost is also factored in. The groups must experiment with how to best maximize the flow of the water while still creating the optimum level of purity, all while keeping cost down. To do this, the groups often explore recycling common products, such as water bottles, to use as casings for the filters. Tinkering with the size of the bottle is normally the first change the groups make to improve flow. One group of students this past year even tried using clay flower pots. They thought the clay would add another level of filtration to the purification process.
Once the groups settle on the casing for their filters, they begin to experiment with the components. It’s always fun to see how the groups determine which layer of material goes on top or how much of each layer is needed. A large amount of raw materials are supplied for use by all the groups, but at some point, those materials may run out. Old designs get torn apart, and the groups start to reuse and recycle some of their past attempts.

Most of the initial trial runs the groups perform fail. Many tweaks are made to refine the initial designs. Failures that have to be worked around and resolved include: having too large of a layer of the smaller grains (such as sand or charcoal), putting the layers in the wrong order, not rinsing the charcoal before putting in the filter, or needing to adjust filter components so that the contents stay in the filter (typically a cotton ball does not stay in place or a coffee filter might tear from the weight of the layers).
To test the filters, the groups are provided with a concoction of water mixed with their group’s expected contaminants. Many groups have the same contaminants to worry about, so typically a base “bad water” is prepared that can be modified to meet the specific needs of each group. The recipe for the “bad water” includes the addition of iron filings, sodium nitrate, sodium phosphate, dirt, and grass clippings to tap water. Some groups identify pH as a problem, so the bad water is adjusted to make it more acidic or basic. If a group has to worry about oil, then vegetable oil is added.
Groups are given one trial run with the “bad water.” Considering that their initial tests were run with tap water, having a more substantial contaminant present (such as soil) can clog their filters. Upon completing modifications to their filters, the groups compete in a final challenge to determine who can design the best filter. The challenge includes a timed filtration period. Then the groups perform a water quality index assessment to determine their water’s purity. Using techniques established in an earlier water quality index project, each group tests the quality of the starting water source and their purified water.
An evaluation of the results compares the starting material (bad water) versus the purified water. The groups calculate what percentage of the contaminants were removed. The calculations also include how effective each layer is in removing the contaminants. Because of the initial research, each layer in the filter has a purpose when it comes to purification. Each group compares and contrasts the amount of each layer to the amount of purification performed. This allow groups to understand their filter’s effectiveness.
The final factoring the groups have to do is based on cost. Armed with the volumes of the materials they used to create these filters, the groups must determine what the total cost is for each filter. Many of the resources needed are quite cheap (like sand and gravel) and the total cost of the filter might be mere pennies to complete. Part of this challenge also includes determining how many filters you could send to this foreign country if you had $1,000 to spend. Now, the issue of shipping costs plays into the planning process.
The water filter project closes with the groups sharing their results during presentations to their classmates. Each group needs to discuss the whole process. They must share their identification of the problem, their exploration into possible solutions, how they came about creating their filters, what processes they used to refine their ideas, and what their results were. The final component they need to share is what they would do next time to improve the process. Most of the time, the groups profess that more initial research would have been beneficial.
While none of the ideas from this lesson have been applied to the larger community, in 2015, students took their newfound passion to improve the world to Chelmsford’s administration. Several groups of students applied for grants through EcoRise and received $1,500 toward the installation of filtered water bottle refill stations throughout the school.
This initiative has inspired other students to become more proactive. Chelmsford has since started two environmental science-based after school groups. Students have applied for and received grants to improve other areas of the school, including adding gardens and improving energy efficiency and classroom air quality.
By challenging students to produce clean water, this water quality unit opens their eyes to the process of discovery. They learn how to use engineering and design to address sustainability issues. They learn they can have an impact on their own community, and the world. At the end of the day, students are authentically engaged, learning techniques that will build their leadership skills, practical skills, and global competency.
Author Bio
Brian Acheson has been teaching College Prep and Honors Biology, Biotechnology, and Environmental Science at Chelmsford High School in Chelmsford, Massachusetts since 2008. He has advanced degrees from the University of Massachusetts, Lowell in Biology, Bioprocessing and Bioengineering, and Biotechnology. Brian is an active participant in many professional development circles, including EcoRise, Fullbright Teachers for a Global Classroom, Amgen Biotech Experience, and Harvard University’s LabXchange, among others. He has worked to receive multiple grants for his school and was awarded the Massachusetts Secretary of Energy and Environment Award for Excellence in Energy and Environmental Education in 2014.